Background
The Acute Leukemia Advocates Network (ALAN) conducted a multi-country survey to gather information on the experiences, quality of life (QoL) and symptoms of adults (16+) with acute leukemia [acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL) and acute promyelocytic leukemia (APL)].
Aims
To examine which factors are most associated with differences in QoL and symptom burden (measured by HM-PRO scores), focusing here on age and patient-reported experience.
Methods
This survey comprised 99 items, designed from a literature review of QoL and acute leukemia followed by input from clinical and patient advocacy experts. The study material was translated (9 languages) and promoted via patient advocacy groups from March 1, 2019 to November 29, 2019.
HM-PRO, an instrument designed to measure patient-reported outcomes in patients with hematological malignancies, was incorporated into the study for assessing QoL and symptoms. This consists of: Part A (impact/QoL); and Part B (signs and symptoms). A higher score in each part represents greater (negative) impact on QoL and symptom burden.
Q6 of the survey asked respondents to report their year of birth. Q9 of the survey provides a measure for disease state: undergoing treatment, in remission following treatment or relapsed following treatment.
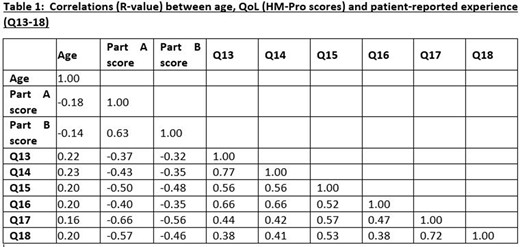
It was hypothesised that patients with a worse experience in each of the following areas would report a worse overall QoL: management by healthcare professional of physical symptoms and side effects (Q13) and emotional impact (Q14); physical and mental health (Q15), information from healthcare professionals (Q16), ability to perform meaningful activities (Q17) and well-being of carers, friends and family (Q18).
Spearman correlation analysis was used to determine the direction and strength of relationships between the measures. Wilcoxon rank-sum, Chi-squared and Kruskal-Wallis rank test were used to examine the differences between groups. Multivariable linear regression, with interaction terms was used to investigate the relationships between the questions and the tumour groups.
Results
There were 552 respondents. Patients self-reported their acute leukemia diagnosis - 332 AML, 139 ALL and 81 APL. There was no overall significant difference between Part A (p=0.55) and Part B (0.23) HM-PRO scores for different acute leukemia types.
Of these, 158 were aged 16-40, 240 were 41-60, 150 were 61-87 and 4 did not answer this question. The results suggest a significant difference across the age groups in both Part A (p=0.005) and Part B (p=0.03) of the HM-PRO scores and signs and symptoms in with younger patients reporting a worse QoL.
The results also confirmed our hypothesis that those with worse scores for Q13-Q18 have a worse QoL (higher HM-PRO score). The correlations (negative) were all statistically significant (p < 0.05), suggesting that good experiences are associated with low HM-PRO scores and vice versa (Table 1).
The predictive value of Q17 and Q18 on overall QoL (HM-PRO scores) varied by tumour type, with a weaker relationship shown for the ALL group. This was confirmed as significant by the multivariable analysis.
Q13-Q16 factors mostly appeared to have similar strengths of relationship with the QoL scores between the different acute leukemia types.
Responses to Q14 differed significantly between disease types (p=0.01) with median 6 for APL vs 7 for ALL and 8 for AML. Similar differences were observed for Q13.
Conclusions
The results confirm there are significant differences (as measured by HM-PRO) in both Part A (impact/QoL) and Part B (signs and symptoms) across age groups, with younger acute leukemia patients reporting a worse QoL. Patients with worse reported experience (Q13-18) have worse HM-PRO scores, suggesting that improving support in these areas may enhance overall QoL.
Pemberton-Whiteley:Celgene: Consultancy, Other; Leukaemia Care: Current Employment; Acute Leukemia Advocates Network: Consultancy, Membership on an entity's Board of Directors or advisory committees; CML Advocates Network: Membership on an entity's Board of Directors or advisory committees; Blood Cancer Alliance: Membership on an entity's Board of Directors or advisory committees; WECAN (Workgroup of European Cancer Patient Advocacy Networks): Consultancy, Membership on an entity's Board of Directors or advisory committees; European Cancer Organisation - Patient Advisory Committee: Membership on an entity's Board of Directors or advisory committees; Patient Evidence: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Other: Organisational Grant Funding; Agios: Other: Organisational Grant Funding; Amgen: Consultancy, Other: Organisational Grant Funding; Astellas: Other; Bristol-Myers Squibb: Consultancy, Other, Speakers Bureau; Daiichi Sankyo: Other; Gilead: Other; Incyte: Consultancy, Other; Jazz: Other, Speakers Bureau; Janssen: Consultancy, Other; Kyowa Kirin: Other; Novartis: Consultancy, Other, Speakers Bureau; Pfizer: Consultancy, Other, Speakers Bureau; Servier: Consultancy, Other; Takeda: Other. Nier:Acute Leukemia Advocates Network: Consultancy; Servier: Consultancy. Geissler:BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding, Speakers Bureau; Incyte: Research Funding; Takeda: Research Funding; Gilead: Consultancy, Speakers Bureau; Biomarin: Consultancy; Janssen: Consultancy, Speakers Bureau; Roche: Consultancy; Servier: Consultancy; UCB: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Wintrich:Takeda: Consultancy, Other: Project funding in MDS; Incyte: Consultancy, Other: Project funding in MDS; Novartis: Consultancy, Other: Project funding in MDS; Janssen: Consultancy, Other: Project funding in MDS; Celgene: Consultancy, Other: Project funding in MDS; Silence Therapeutics: Consultancy, Other: Project funding in MDS. Verhoeven:Novartis: Consultancy; Janssen: Consultancy. Christensen:Autisme 4700 DK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Other: Organisational Grant Funding; Bristol Myers Squib: Consultancy; Gilead: Other: Organisational Grant Funding; Incyte: Consultancy, Other: Organisational Grant Funding; Janssen: Consultancy, Other: Organisational Grant Funding; Novartis Nordic: Consultancy, Other: Organisational Grant Funding; Pfizer: Other: Organisational Grant Funding; Roche: Consultancy, Other: Organisational Grant Funding; Takeda: Consultancy, Other; LYLE: Consultancy, Membership on an entity's Board of Directors or advisory committees. Salek:Merck: Consultancy; Agios: Consultancy, Honoraria; Pfizer: Honoraria, Speakers Bureau. Oliva:Amgen: Consultancy; Abbvie: Consultancy; Apellis: Consultancy; Alexion: Consultancy; Novartis: Consultancy; BMS: Consultancy, Honoraria, Patents & Royalties, Speakers Bureau. Ionova:Takeda: Research Funding; BMS: Research Funding. Bradley:Quality Health Ltd: Current Employment. Tate:Quality Health Ltd: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal